 |
SEOUL, South Korea, Oct. 24, 2023 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, today announced a significant breakthrough in the treatment of non-small cell lung cancer (NSCLC) with resistance to certain targeted therapies.
A groundbreaking study, conducted by Samsung Medical Center and utilizing Lunit SCOPE IO, showcases the efficacy of atezolizumab plus bevacizumab and chemotherapy for NSCLC patients with EGFR or ALK mutations. The study result was concurrently published in the Journal of Clinical Oncology (JCO, IF 50.739) and presented in an oral presentation at the ESMO (European Society of Medical Oncology) 2023 Annual Meeting. Against the backdrop of growing interest and need for an AI biomarker in medical practices, this marks the second time a study utilizing Lunit SCOPE IO has been published in the prestigious JCO.
This research marks a turning point for NSCLC patients with EGFR or ALK mutations, as prior trials faced challenges in identifying effective treatments. Patients with these mutations often respond favorably to targeted agents, but over time, the effectiveness of these treatments diminishes. Multiple leading pharmaceutical companies have attempted clinical trials of immunotherapy in this setting, which have not succeeded to date. This underscores the significance of this first successful phase III trial in this setting, successfully combining immunotherapy with chemotherapy and improving clinical outcomes.
The study enrolled a total of 228 NSCLC patients with activating EGFR mutation (215 patients) or ALK translocation (13 patients), who experienced progression after the relevant tyrosine kinase inhibitor (TKI) therapy. Patients were randomized into two arms: the ABCP arm (atezolizumab plus bevacizumab/paclitaxel/carboplatin) and the PC arm (pemetrexed plus carboplatin or cisplatin). The results demonstrated the superiority of the ABCP arm, with significantly higher objective response rates (ORR; 69.5% vs. 41.9%) and a longer median progression-free survival (PFS; 8.48 months vs. 5.62 months).
Lunit SCOPE IO, an AI-powered TIL analyzer for assessing immune phenotype from H&E, played a pivotal role in this research. By assessing patients' immune phenotype and predicting response to immunotherapy, Lunit SCOPE IO enabled the identification of individuals more likely to benefit from ABCP arm treatment. In the group with an Inflamed Score below 20%, there was no significant difference in PFS between ABCP arm and PC arm (8.28 months vs. 6.93 months). However, a substantial difference in PFS was observed in the group with an Inflamed Score of 20% or higher (12.91 months vs. 4.86 months), surpassing the overall study group. Immune phenotype as assessed by Lunit SCOPE IO showed predictive power in stratifying patients more likely to respond to ABCP treatment.
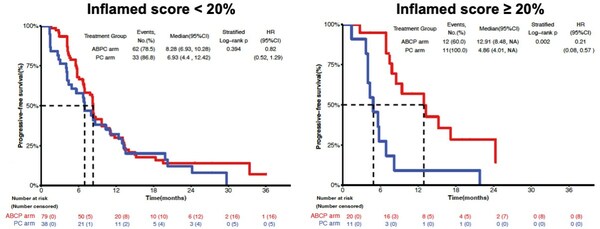
Lunit SCOPE IO demonstrated that the patient group with an Inflamed Score of 20% or higher showed longer Progression-free survival when treated with ABCP arm
"The study's acceptance by the JCO and ESMO underlines the significance of our contribution to the field of lung cancer treatment. Lunit SCOPE IO's ability to quantitatively assess immune phenotype, enabling its use as a biomarker for immunotherapy, has the potential to meaningfully improve the clinical use of immunotherapy. In this case, by identifying those who will benefit most from atezolizumab plus bevacizumab, we aim to enhance the accessibility and effectiveness of this treatment," said Brandon Suh, CEO of Lunit. "We aim for Lunit SCOPE IO to continue to make immune phenotyping a quantitative biomarker readily accessible for research, clinical use, and companion diagnostics (CDx) business."
About Lunit
Lunit is a deep learning-based medical AI company on a mission to conquer cancer. Our focus is on developing AI solutions for precision diagnostics and therapeutics, ensuring the right diagnosis, and treatment, at the right cost for each patient. Lunit is devoted to developing advanced medical image analytics and AI-based biomarkers via cutting-edge technology.
Founded in 2013, Lunit has been acknowledged around the world for its advanced, state-of-the-art technology and its application in medical images. As a medical AI company grounded on clinical evidence, the company's findings are presented in major peer-reviewed journals, such as the Journal of Clinical Oncology and JAMA Network Open, and global conferences, including ASCO and AACR.
After receiving FDA clearance and the CE Mark, our flagship Lunit INSIGHT suite is clinically used in approximately 2,000+ hospitals and medical institutions across 40+ countries. Lunit is headquartered in Seoul, South Korea, with offices and representatives worldwide. For more information, please visit lunit.io
About Lunit SCOPE
Lunit SCOPE is a suite of AI-powered software that analyzes tissue slide images for digital pathology and AI biomarker development, aiming to optimize workflow and facilitate more accurate and predictive clinical data for clinicians and researchers.
Lunit SCOPE platform offers multiple AI-powered tissue analysis products and assays that can streamline digital pathology workflow and diagnostics and enhance the drug development process.
Lunit SCOPE IO analyzes the tumor microenvironment (TME) based on H&E analysis and provides AI-based predictive clinical outcome information. In addition, AI-driven Immunohistochemistry (IHC) slide analysis services are offered, through products such as Lunit SCOPE PD-L1, Lunit SCOPE HER2, Lunit SCOPE ER/PR, and others.
 1 year ago
569
1 year ago
569 






 English (United States)
English (United States)