SEOUL, South Korea, Oct. 23, 2023 /PRNewswire/ -- VUNO Inc., South Korean medical AI company has received 510(k) clearance from the Food and Drug Administration (FDA) for its AI-powered brain quantification device, VUNO Med®-DeepBrain®.
VUNO Med®-DeepBrain® is intended to automate the current manual process of identifying, labeling, and quantifying segmentable brain structures from MRI images. The AI software efficiently provides volumetric data on over 100 brain regions through brain parcellation and provides cortical thickness, and white matter hyperintensity (WMH). The patients' atrophy data is compared with a normal population and displayed with a normative percentile measurement. This high quality, quantifiable brain data can be compiled and presented as a customizable report to clinicians, which may be valuable in dementia and other neurodegenerative diseases.
With the FDA clearance in hand, VUNO intends to bolster its sales and marketing efforts targeting US medical institutions. Furthermore, the company plans to enhance its collaborations with globally recognized pharmaceutical firms seeking AI-based brain MRI quantification technology.
VUNO has previously explored the potential for early diagnosis of Alzheimer's disease with information provided by VUNO Med®-DeepBrain® through clinical research. According to clinical research presented at the Alzheimer's Association International Conference (AAIC) held in July 2023, VUNO Med®-DeepBrain® demonstrated its ability to provide information that can be used to predict amyloid positivity in patients experiencing Subjective Cognitive Decline (SCD), one of the earliest noticeable symptoms of Alzheimer's disease and related dementias.
This suggests that the product can contribute to the early detection and management of patients who may progress to dementia even before Mild Cognitive Impairment (MCI) or early dementia.
Yeha Lee, CEO of VUNO said "VUNO Med®-DeepBrain® marks the first FDA clearance from VUNO, and we expect it will be a steppingstone for VUNO's expansion into the U.S. market," adding "with this product, we will make every effort to help improve the declining quality of life experienced by many dementia patients."
About VUNO Inc.
VUNO is a Seoul-based leading AI medical software company that applies deep learning to develop data-driven AI medical solutions using medical imaging and biosignal. We strive to present a whole new level of experience to medical practitioners in their day-to-day workflows, empowering them to make better diagnostic decisions faster and more accurately and to provide quality patient care and treatment planning to patients. For more information, visit www.vuno.co.
 1 year ago
241
1 year ago
241 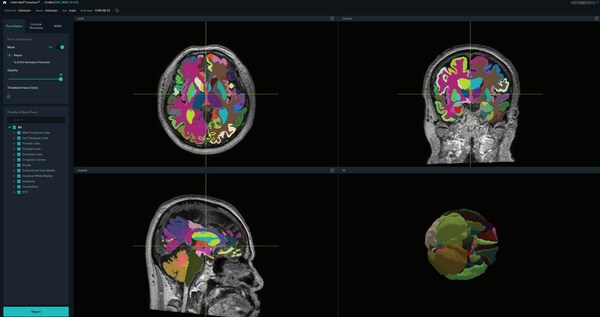






 English (United States)
English (United States)